Clémence Lacroix*, Isabelle Alleman-Brimault, Arnaud Zalta, Frank Rouby,
Catherine Cassé-Perrot, Elisabet
APHM, INSERM, Inst Neurosci Syst, UMR 1106, Aix Marseille Univ, University Hospital Federation DHUNE, Service de Pharmacologie Clinique et Pharmacovigilance, Marseille, France
Medical use of cannabis has been receiving growing attention over the last few decades in
modern medicine. As we know that the endocannabinoid system is largely involved in
neurological disorders, we focused on the scientific rationale of medical cannabis in three
neurological disorders: amyotrophic lateral sclerosis, Parkinson’s disease, and
Alzheimer’s disease through pharmacological plausibility, clinical studies, and patients’
view. Clinical studies (randomized controlled trials, open-label studies, cohorts, and case
reports) exploring medical cannabis in these disorders show different results depending on the methods and outcomes. Some show benefits on motor symptoms and others on nonmotor symptoms and quality of life. Concerning patients’ view, several web surveys were collected, highlighting the real use of cannabis to relieve symptoms of neurological
disorders, mostly outside a medical pathway. This anarchic use keeps questioning
particularly in terms of risks: consumption of street cannabis, drug–drug interactions
with usual medical treatment, consideration of medical history, and adverse reactions
(psychiatric, respiratory, cardiovascular disorders, etc.), underlining the importance of a
medical supervision. To date, most scientific data support the therapeutic potential of
cannabis in neurological disorders. As far as patients and patients’ associations are calling
for it, there is an urgent need to manage clinical studies to provide stronger evidence and
secure medical cannabis use.
INTRODUCTION
Medical use of cannabis has been receiving growing attention over the last few decades in modern medicine. As cannabis is a complex plant containing hundreds of cannabinoids, we keep questioning about its therapeutic benefits, justified by its pleiotropic pharmacological activity. As a result, it has been reported that changes in endocannabinoid levels may be related to neurological diseases such as
Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, and multiple sclerosis (Fraguas-Sánchez and Torres-Suárez, 2018). As we know that the endocannabinoid system (ECS) is largely involved in neurological disorders, we here chose to focus on the scientific rationale of medical cannabis through a narrative review in three neurological disorders: amyotrophic lateral sclerosis(ALS), Parkinson’s disease (PD), and Alzheimer’s disease (AD) through pharmacological plausibility, clinical studies, and patients’ view. Developing medical cannabis could be an important issue to better control neurodegeneration.
PHARMACOLOGICAL PLAUSIBILITY
ECS is largely expressed in the cerebellum, basal ganglia, and hippocampus and is thus an area of choice for molecular targets. Characterization of the ECS and detection of widespread cannabinoid receptors in the brain and peripheral tissues have opened the door to a vast field of research. The ECS is formed by cannabinoid receptors 1 and 2 (CB1 and CB2), the two endocannabinoids anandamide and 2-arachidonoylglycerol, and endocannabinoid anabolic and catabolic enzymes. Manipulation of the ECS may have beneficial diseasemodifying potential in neurological disorders. Exogenous cannabinoids play a pleiotropic activity mostly through two cannabinoid receptors: CB1 is predominantly expressed in the brain, and CB2 is primarily found in the cells of the immune system (Lucas et al., 2018). Since the pathophysiology of motor neuron degeneration in ALS may involve mitochondrial dysfunction, excessive glutamate activity, oxidative stress, neuroinflammation, and growth factor deficiency, cannabis could be effective in modulating these processes (Bilsland and Greensmith, 2008; Carter et al., 2010; Papadimitriou et al., 2010; Appel et al., 2011). To support these hypotheses, a recent metaanalysis of preclinical studies in murine ALS models conducted by Urbi and colleagues suggests that cannabinoid receptor agonists may improve survival time (Urbi et al., 2019b).
PD mostly involves dopaminergic and cholinergic systems. The interactions between cannabinoids and dopamine in the basal ganglia may involve both the modulation of other neurotransmitters (GABA, glutamate) and the activation of CB1 and CB2 (Stampanoni Bassi et al., 2017; Patricio et al., 2020). Preclinical studies in the animal model of PD have shown various influences of cannabis on motor and non-motor behaviors: reducing motor fluctuations and levodopa-induced dyskinesias (Segovia et al., 2003; Morgese et al., 2007; Song et al., 2014). Activation of CB2 has shown a reduction in dopamine depletion in PD rats (García-Arencibia et al., 2007). In a preclinical study investigating the role of a CB2 receptor agonist on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in a mouse model of PD managed in 2017, the use of a CB2 agonist reversed the depletion of CB2 and thus increased the levels of dopamine and improved the behavior of PD mice (Shi et al., 2017). Cannabinoids seem to be protective by binding to the CB1 receptor, inhibiting the dopamine beta hydroxylase activity and decreasing glutamate levels or by binding to CB2, reducing neuroinflammation (Ferreira et al., 2020). All these considerations suggest therapeutic benefits of cannabis in PD.AD is characterized by extracellular deposits of β-amyloid plaques and neurofibrillary tangles of tau protein (Selkoe, 2011). Cannabis promotes neuroprotection through different signal pathways mediated indirectly by CB receptors by reducing the β-amyloid peptide action and tau phosphorylation, as well as modulating oxidative stress and inflammation (Esposito et al., 2006; Aso and Ferrer, 2014). CB1 and CB2 agonists ameliorated memory and cognitive impairment in mice that have received intracerebral injection of β-amyloid peptide (Ramirez, 2005). CB2 activation also reduced levels of neurotoxic factors and pro-inflammatory mediators produced by reactive astrocytes and microglial cells, stimulated microglial proliferation and migration, and decreased β-amyloid peptide levels (Cristino et al., 2020). To resume, cannabis improved immune function, amyloidogenesis, and reduced behavioral symptoms and pain but also stimulated appetite and inhibited acetylcholinesterase in animal models of AD (Cooray et al., 2020; Li et al., 2020).
CLINICAL STUDIES
We managed a literature search on Medline using the keywords “medical cannabis” and “neurological disorders,” “medical cannabis” and “amyotrophic lateral sclerosis,” “medical cannabis” and “Parkinson,” and “medical cannabis” and “Alzheimer”. The articles were thoroughly screened by reviewing each article with titles, abstracts, and content of the full articles. We only included the studies published between 1986 and 2021 and human studies (clinical trials, case reports, and published protocols) in the English language, including adults of 18 years of age and older, and we excluded review articles and position studies (Figure 1). An additional search on clinicaltrials. gov was also performed using “ALS” and “cannabis,” “Parkinson” and “cannabis,” and “Alzheimer” and “cannabis”. Only sparse data on the benefits of medical cannabis in neurological disorders are available from clinical studies. As we know that cannabis is a complex plant with hundreds of phytocannabinoids, several components are studied in the following clinical studies. Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most studied in therapeutic use as These pharmacological considerations concerning cannabis in neurological disorders suggest mechanism-based therapeutictargets for future clinical studies.
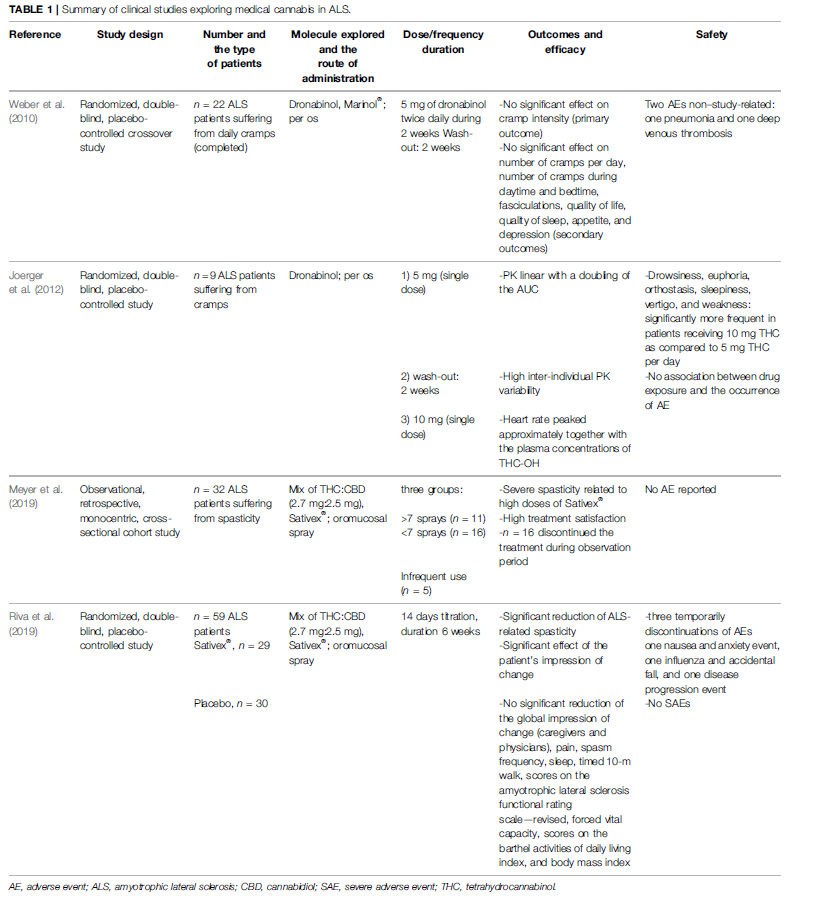
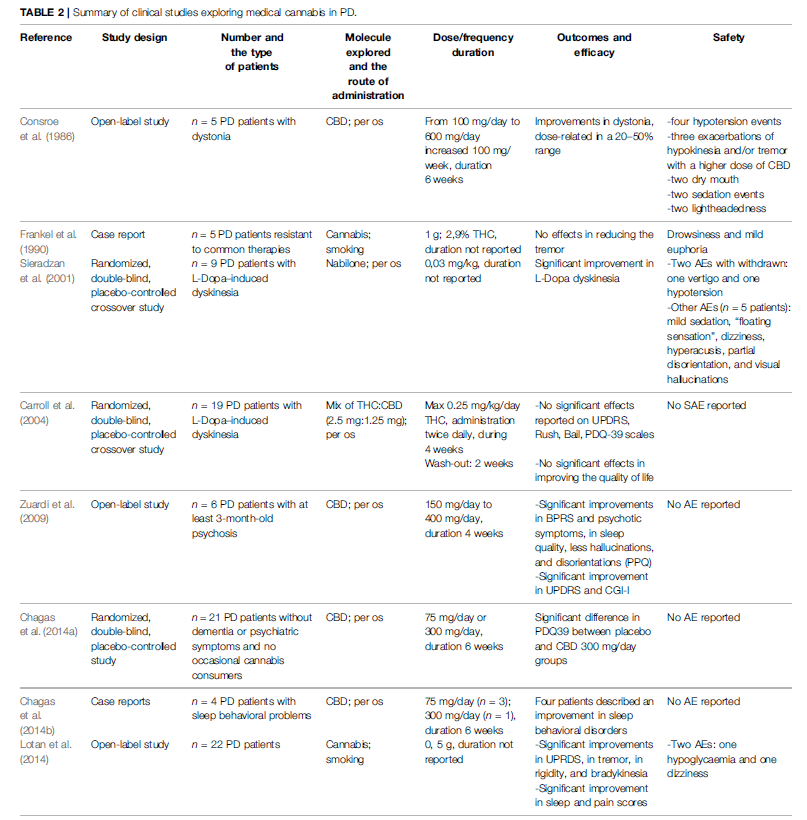
To date, we have only found four clinical studies exploring the use of medical cannabis in ALS (Weber et al., 2010; Joerger et al., 2012; Meyer et al., 2019; Riva et al., 2019). Data from these studies are summarized in Table 1. The use of dronabinol alone did not demonstrate improvement in cramp intensity, cramp frequency, and fasciculation intensity neither on quality of life, sleep, appetite, and depression in a randomized controlled trial (RCT) of 2010 (Weber et al., 2010). The lack of treatment effect could be due to the short duration treatment (2 weeks). In parallel, an equilibrated mix of tetrahydrocannabinol (THC) and cannabidiol (CBD) in the oromucosal spray Sativex® seems to be effective on ALS-related spasticity and on the patients’ global impression of change in a 6-week RCT (Riva et al., 2019) and also in a cohort study (Meyer et al., 2019). Noticeably, Sativex® (an equilibrated mix of THC and CBD) is already commercialized and indicated for symptom improvement in adult patients with resistant spasticity due to multiple sclerosis. All these studies reported good tolerability of medical cannabis. Therefore, these modest but encouraging results suggest the need for further studies enrolling a higher number of patients. Concerning PD patients, eight clinical studies were published (Consroe et al., 1986; Frankel et al., 1990; Sieradzan et al., 2001; Carroll et al., 2004; Zuardi et al., 2009; Chagas et al., 2014a, 2014b; Lotan et al., 2014), as shown in Table 2. Medical cannabis could be effective both on motor symptoms (dystonia, dyskinesia, and fluctuations), and non-motor symptoms (anxiety, sleep quality, hallucinations, and disorientation) (Consroe et al., 1986; Frankel et al., 1990; Sieradzan et al., 2001; Zuardi et al., 2009; Chagas et al., 2014a; Lotan et al., 2014). Two studies (one RCT and one case report of five PD patients) show that there is not any reduction of motor and non-motor symptoms. One open-label study shows that there is an improvement of motor and non-motor symptoms only at the highest dose of CBD (400 mg/day for 4 weeks). Case reports of four PD patients show that there is an improvement of the quality of sleep without nightmares and reduction of agitation. Five studies show improvement of motor and nonmotor symptoms and quality of life (three open-label studies and two RCTs). Anyway, all studies demonstrate that there are no serious adverse effects. The main limitations to these findings are short study duration and small sample sizes. Another limitation may be due to the low bioavailability of THC and CBD in oral preparations. This means that there is an obvious need for larger well-conducted studies.
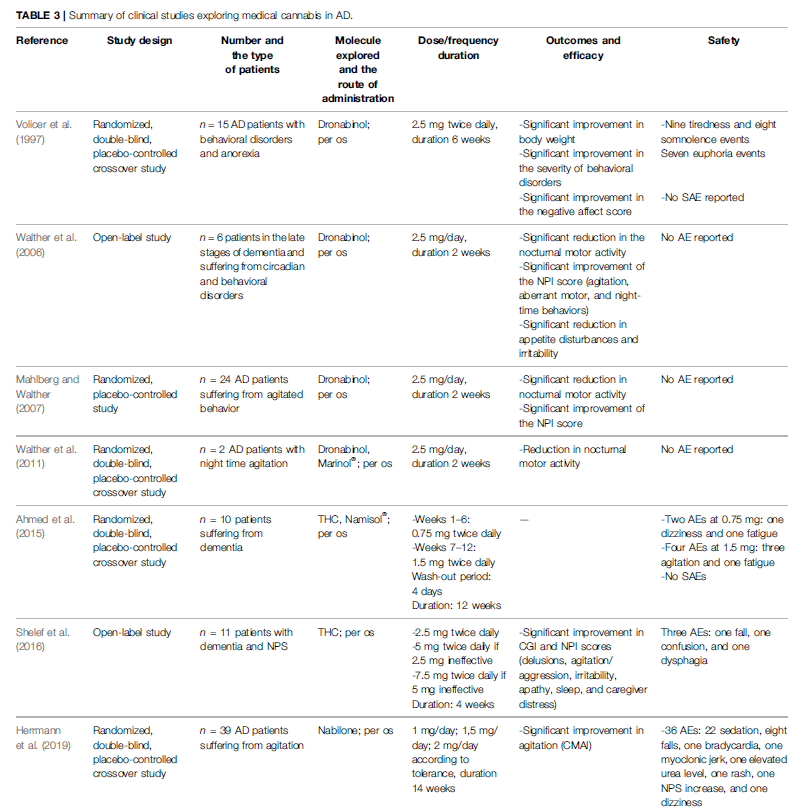
To our knowledge, five RCTs and two open-label studies were published in AD regarding medical cannabis effectiveness and safety (Volicer et al., 1997; Walther et al., 2006; Mahlberg and Walther, 2007; Walther et al., 2011; Ahmed et al., 2015; Shelef et al., 2016; Herrmann et al., 2019). Results of these studies are available in Table 3. Only dronabinol and nabilone were experimented in AD patients. The benefits published in these studies were improving in agitation, nocturnal motor activity, disturbed behavior, anorexia, and the patient’s global impression of change (Volicer et al., 1997; Walther et al., 2006; Walther et al., 2011; Shelef et al., 2016; Herrmann et al., 2019). To resume, among these 19 clinical studies, nine were randomized double-blind placebo-controlled designed. Openlabel design has inherent limitations of a placebo effect and rater bias. Moreover, as the experimental products and the routes of administration used were different (synthetic or natural and mix of cannabinoids or only one cannabinoid; per os or smoked), it adds an additional difficulty to compare results. According to the experimental product, it could also be difficult to perform a placebo-controlled design because of the conspicuous and characteristic smell of a cannabis cigarette, for example. It still underlines that more well-conducted studies would be necessary to further strengthen evidence of effectiveness. Nevertheless, these results are hopeful for patients suffering from these neurological disorders. Moreover, adverse effects reported with the use of medical cannabis do not seem to be limiting for its clinical use. Reported adverse effects were expected ones compared to the knowledge of cannabis use in general population (drowsiness, euphoria, sleepiness, weakness, dizziness, hypotension, and dry mouth). Due to pharmacokinetics variability of medical cannabis (and its numerous metabolites), future studies should apply parallel group study design rather than crossover design. To date, 13 studies are registered in clinicaltrials.gov; two protocols are already published in Medline (Urbi et al., 2019a; Timler et al., 2020). Urbi et al. published a protocol of a randomized double-blind placebo-controlled study in ALS patients to evaluate the efficacy of a mix oil of CBD:THC (25 mg CBD: <2 mg THC) in slowing the disease progression. Secondary objectives are safety and tolerability. Timler et al. carried a randomized double-blind crossover study experimenting a mix oil of THC:CBD (3:2) in patients with dementia, on behavior symptoms, quality of life, and discomfort by pain. Overall, clinical studies exploring medical cannabis in neurological disorders show different results depending on the methods and outcomes. Some show benefits on motor symptoms of neurological diseases, some on non-motor symptoms, and others no benefit at all. Therefore, it is becoming essential to conduct more and larger clinical studies in order to scientifically enlighten clinicians and first and foremost patients.
WHAT ABOUT PATIENTS’ VIEW?
As cannabis has been presented as a treatment for many medical conditions for few years, patients experiment this plant in many ways to manage their neurological disorders. Nevertheless, very few surveys have been conducted to describe 1) the consumers (medical condition and demographics); 2) the consumption (the cannabinoid type, form, route of administration, frequency, duration, and way of acquisition); 3) the relief symptoms (duration and level of the relief); 4) the adverse effects (type, duration, and frequency). It is unavoidable to understand the motivations and experiences of cannabis use among people living with neurological disorders to better orient clinical trials. In 2004, Amtmann and colleagues published a worldwide anonymous web survey analyzing the answers of 131 ALS patients (Amtmann et al., 2004). The mean age was 54 years, and patients were mostly male (75%). Respondents reported a stable family life and a high education level for the majority. The median time since ALS diagnosis was 3 years, and the mean duration was 4 years. About 10% of the respondents (n = 13) reported the use of cannabis to relieve symptoms of ALS in the last 12 months. They mostly consume smoking cannabis. Only three of them reported using medical cannabis (dronabinol).
Concerning relieve symptoms, patients reported cannabis as moderately effective in symptoms of appetite loss, depression, pain, spasticity, and drooling and ineffective in reducing difficulties with speech and swallowing and sexual dysfunction.
The longest relief was reported for depression. In 2004
Venderova and colleagues also sent an anonymous questionnaire to all patients attending the Prague Movement Disorder Centre (Venderová et al., 2004). In total, 339 questionnaires were returned, and 25% of the respondents declared having taken cannabis. The mean age of cannabis users was 63.9 years, and the mean duration of PD was 8.3 years. They mostly reported an oral consumption once a day. Interestingly, none of them reported their doctor. PD patients described alleviations, especially in motor symptoms: 44.7% in bradykinesia, 37.7% in muscle rigidity, 30% in tremor, and 14.1% in L-dopa-induced dyskinesias. Another anonymous web survey managed on PD patients in the United States in 2020 analyzed the answers of 1,064 patients (Feeney et al., 2021). The mean age of the respondents was 71.2 years, male accounted for 52.5%, and the mean PD duration was 7.4 years. They were mostly highly educated with 78% of retired, evolving in a stable family life. About 25% of them reported the use of cannabis in the previous 6 months, and 35.6% of them considered themselves as regular users. They most frequently reported spraying or drooping, smoking, and eating as their primary method of cannabis use. The ways of acquisition were medical dispensary (38.7%) and family/friend gift (24.5%). When known, patients reported products with a high THC dosage in 21.2%, to get a better efficacy for both motor and non-motor symptoms. The reported relief symptoms were non-motor symptoms insufficiently controlled by classic medications: anxiety (45.5%), pain (44%), sleep disorders (44%), and specific motor symptoms such as stiffness (43%) or tremor (42%). Only 12.6% of PD cannabis users reported adverse effects (anxiety, impaired coordination, and dizziness). Interestingly, cannabis non-users (75.5%) reported two major reasons for not using cannabis: the lack of evidence (59.9%) and the fear of cannabis adverse effects (34.9%). In 2021, a German nationwide questionnaire survey described the used of medical cannabis in PD patients (Yenilmez et al., 2021). A total of 1,348 questionnaires were analyzed. The mean age of the patients was 71.6 years, and the mean PD duration was 11.6 years. Cannabis use was reported in 8.4% of the questionnaires, with a reduction of pain and muscle cramps in more than 40% of users (respectively, 43.9 and 41.4%). Moreover, more than 20% of them described an improvement in depression (28.1%), stiffness/akinesia (27.3%), sleep disorders (27.1%), freezing (25.0%), tremor (24.3%), anxiety (24.0%), and restless legs syndrome (21.4%). The improvements were related to 54.1% of oral CBD use and to 68.2% inhaling THC-containing cannabis.
In the majority of patients (85%), cannabis was well-tolerated. Adverse effects reported were mainly fatigue, dizziness, and ravenous appetite. Another recent survey showed that 95% of movement disorders specialist neurologists reported to be asked to prescribe medical cannabis to their patients (Bega et al., 2017). Concerning AD patients, a recent Polish anonymous web survey addressed to caregivers identified the attitudes and beliefs of caregivers of individuals with AD toward CBD oil in Poland (Leszko and Meenrajan, 2021). A total of 73 caregivers answered the questionnaire. They reported an effective use of CBD oil in behavioral symptoms of AD, to slow memory loss, agitation, anxiety, and insomnia. Most of the caregivers (84%) answered that CBD oil improved their care recipient’s quality of life. None of them reported adverse effects with the use of CBD oil. It is also interesting to note that only 63% of them informed their physician about this habit. In this survey, people also reported lack of information about the legacy, the medical use of cannabis as far as a lack of scientific data. Despite being great sources of information, these surveys present several limitations. First, the results are based on small sample sizes compared to the affected population; respondents may not have been representative of the entire ALS, PD, and AD population. Second, internet users’ population constitutes a selection bias because all patients with neurological disorders could not use the internet, and internet users tend to be highly educated. Third, cannabis users may have been more inclined to answer the surveys and inflated the number of users and benefits, leading to a possible answer bias. Another limitation is the country of survey and/or residence because cannabis could be legal or not.
REFERENCES
Ahmed, A. I., van den Elsen, G. A., Colbers, A., Kramers, C., Burger, D. M., van der
Marck, M. A., et al. (2015). Safety, Pharmacodynamics, and Pharmacokinetics
of Multiple Oral Doses of delta-9-tetrahydrocannabinol in Older Persons with
Dementia. Psychopharmacology (Berl) 232, 2587–2595. doi:10.1007/s00213-
015-3889-y
Amtmann, D., Weydt, P., Johnson, K. L., Jensen, M. P., and Carter, G. T. (2004).
Survey of Cannabis Use in Patients with Amyotrophic Lateral Sclerosis. Am.
J. Hosp. Palliat. Care 21, 95–104. doi:10.1177/104990910402100206
Anderson, L. L., Absalom, N. L., Abelev, S. V., Low, I. K., Doohan, P. T., Martin, L.
J., et al. (2019). Coadministered Cannabidiol and Clobazam: Preclinical
Evidence for Both Pharmacodynamic and Pharmacokinetic Interactions.
Epilepsia 60, 2224–2234. doi:10.1111/epi.16355
Appel, S. H., Zhao, W., Beers, D. R., and Henkel, J. S. (2011). The Microglial-
Motoneuron Dialogue in ALS. Acta Myol 30, 4–8.
Aso, E., and Ferrer, I. (2014). Cannabinoids for Treatment of Alzheimer’s Disease:
Moving toward the Clinic. Front. Pharmacol. 5, 37. doi:10.3389/fphar.2014.
00037
Bega, D., Simuni, T., Okun, M. S., Chen, X., and Schmidt, P. (2017). Medicinal
Cannabis for Parkinson’s Disease: Practices, Beliefs, and Attitudes Among
Providers at National Parkinson Foundation Centers of Excellence. Mov Disord.
Clin. Pract. 4, 90–95. doi:10.1002/mdc3.12359
Bilsland, L. G., and Greensmith, L. (2008). The Endocannabinoid System in
Amyotrophic Lateral Sclerosis. Curr. Pharm. Des. 14, 2306–2316. doi:10.
2174/138161208785740081
Carroll, C. B., Bain, P. G., Teare, L., Liu, X., Joint, C., Wroath, C., et al. (2004).
Cannabis for Dyskinesia in Parkinson Disease: A Randomized Double-Blind
Crossover Study. Neurology 63, 1245–1250. doi:10.1212/01.WNL.0000140288.
48796.8E
Carter, G. T., Abood, M. E., Aggarwal, S. K., and Weiss, M. D. (2010). Cannabis and
Amyotrophic Lateral Sclerosis: Hypothetical and Practical Applications, and a
Call for Clinical Trials. Am. J. Hosp. Palliat. Care 27, 347–356. doi:10.1177/
1049909110369531
Chagas, M. H., Eckeli, A. L., Zuardi, A. W., Pena-Pereira, M. A., Sobreira-Neto, M.
A., Sobreira, E. T., et al. (2014a). Cannabidiol Can Improve Complex Sleep-
Related Behaviours Associated with Rapid Eye Movement Sleep Behaviour
Disorder in Parkinson’s Disease Patients: a Case Series. J. Clin. Pharm. Ther. 39,
564–566. doi:10.1111/jcpt.12179
